Introduction
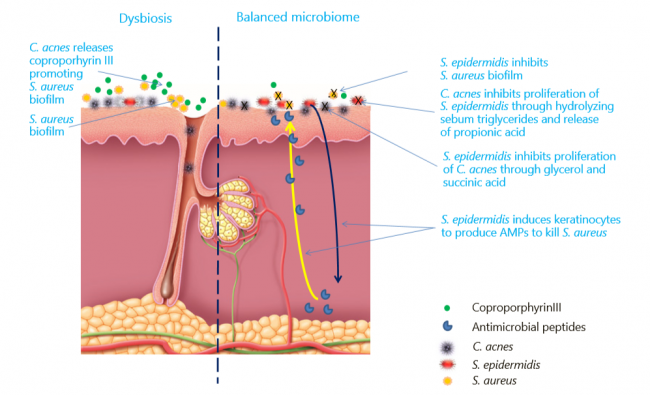
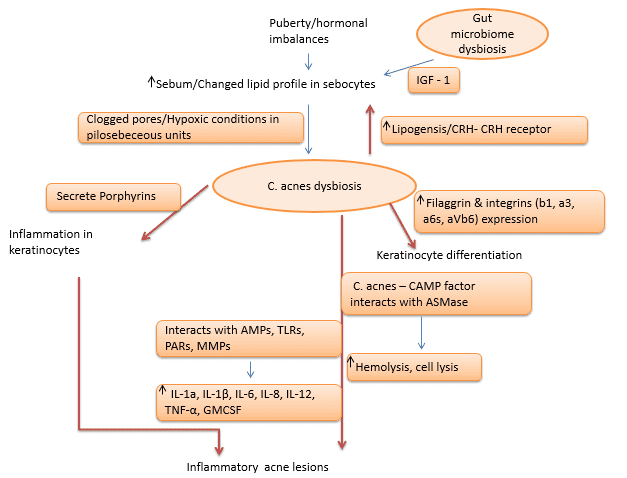
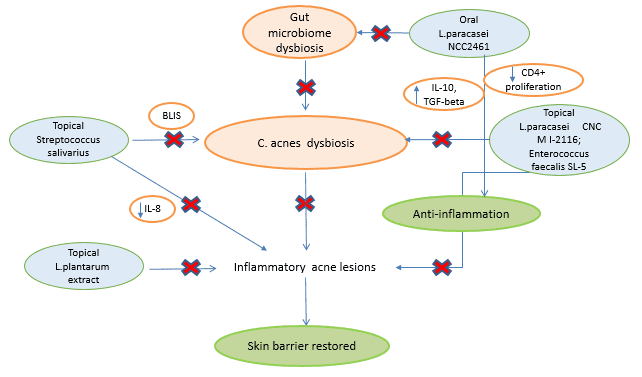
Skin is the home for a vast variety of microorganisms, including archaea, bacteria, fungi, viruses and arthropods, which together make up what is known as skin microbiome[1]. The skin microbiome majorly comprises Actinobacteria (51.8%), Firmicutes (24.4%), Proteobacteria (16.5%) and Bacteroidetes (6.3%)[2]. The microbiome of epithelia and sebaceous follicles comprises Gram-positive bacteria such as Staphylococcus epidermidis and Cutibacterium acnes (C. acnes hereafter), and fungal species such as Malassezia. As skin commensals, C. acnes and S. epidermidis interact with the host, helping protect healthy skin from colonization by pathogens such as Staphylococcus aureus[3], [4]. Any imbalance caused by internal/external factors in the microbiome composition is termed as dysbiosis. The dysbiosis factors mainly include genetic, hormonal changes/imbalances, western diet, overuse of cosmetics/antibiotics and infections[5], [6]. Factors such as the western diet and overuse of antibiotics are also associated with changes in gut microbiome, which, in turn, has effects on skin microbiome via immune modulation, leading to skin ailments such as acne, eczema, psoriasis, rosacea, and keratosis pilaris[7], [8], [9]. This white paper discusses skin microbiome and dysbiosis from the acne perspective.
Types of Acne
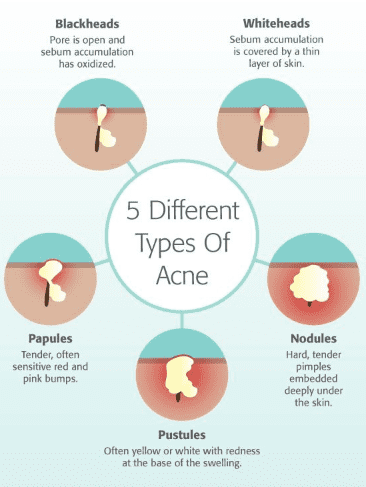
Acne (common term for Acne vulgaris) is a common inflammatory chronic skin problem, affecting the pilosebaceous units of hair follicles. About 85% of adolescents and young adults are estimated to have been affected by this disease. Acne is the second most common dermatological condition after dermatitis[10]. Acne is mainly classified into four types based on the severity of lesions formed on the epidermis: (1) comedonal, (2) papular, (3) pustular and (4) nodules. The comedonal includes the non-inflammatory mild lesions commonly known as black heads and white heads, as shown in fig.1. Papular and pustular are inflammatory mild to moderate lesions, while nodules are the most severe ones[11].