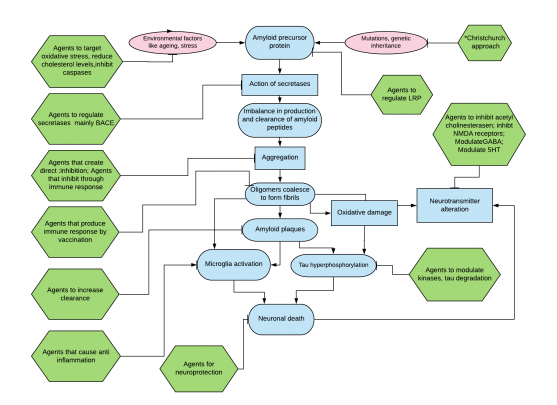
Introduction
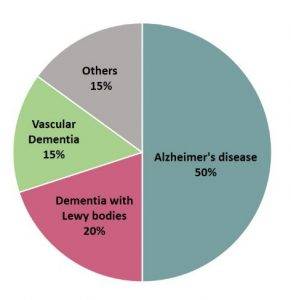
Dementia, a chronic and progressive disorder due to the apoptosis of neurons and worsening of cognitive function, has been posing challenges to caregivers as well as medical professionals. Dementia occurs in different forms such as vascular dementia, Lewy body dementia, Alzheimer’s disease and others.
Common symptoms are memory loss, delusions, hallucinations, violence, depression etc.

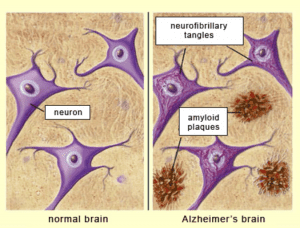
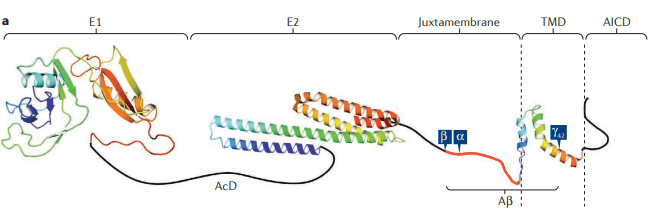
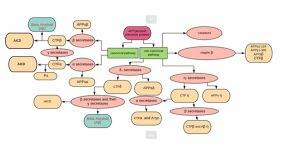
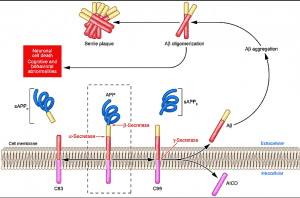
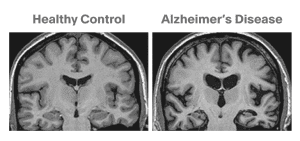
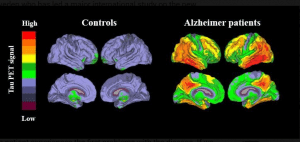
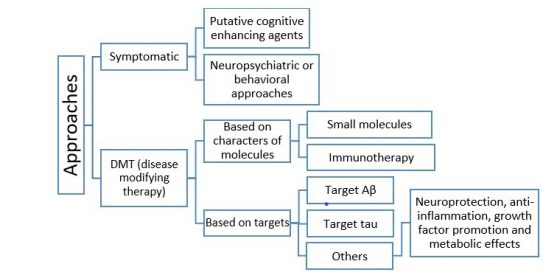
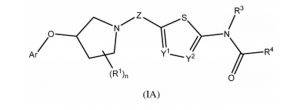 0-Glycoprotein-2-Acetamido-2-Deoxy-3-D-Glucopyranosidase inhibitors deals with pharmaceutical salts involving method of inhibiting 0-GlcNAcase to treat neurodegenerative diseases such as AD
0-Glycoprotein-2-Acetamido-2-Deoxy-3-D-Glucopyranosidase inhibitors deals with pharmaceutical salts involving method of inhibiting 0-GlcNAcase to treat neurodegenerative diseases such as AD