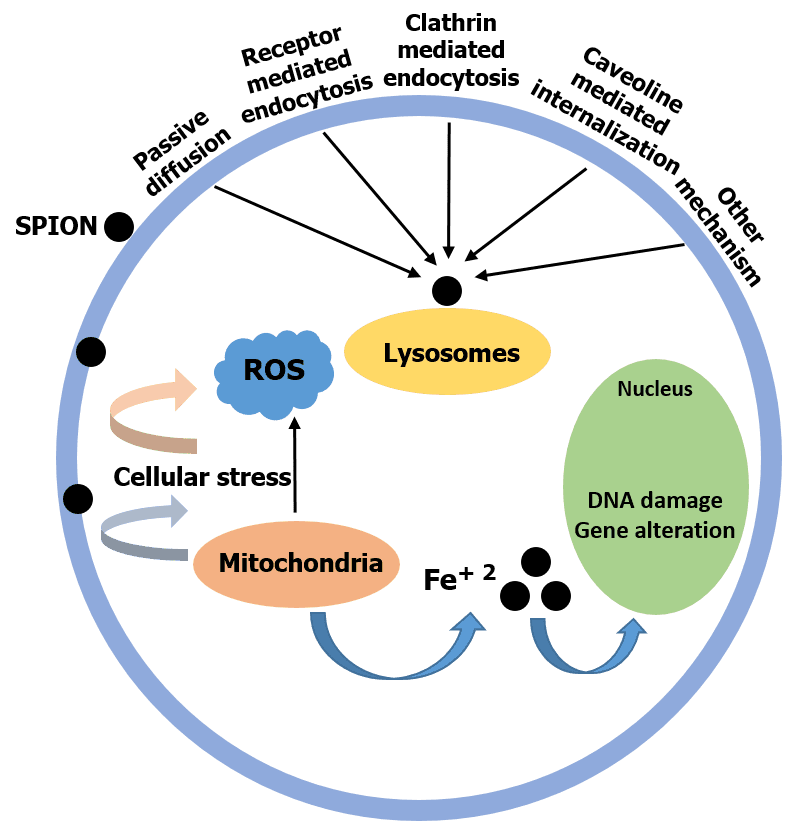
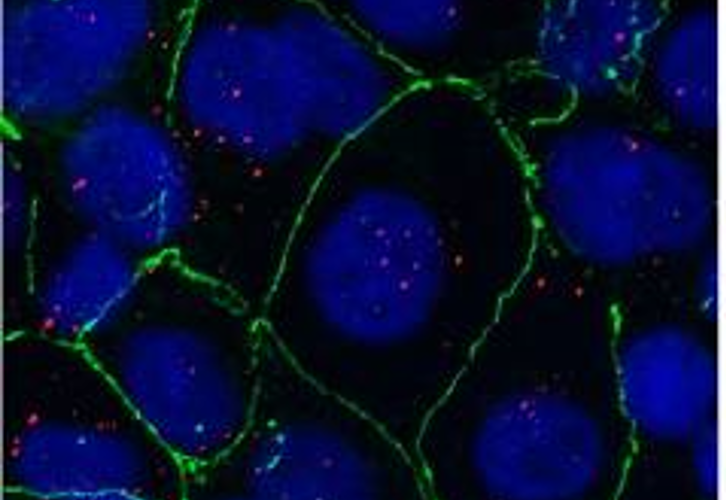
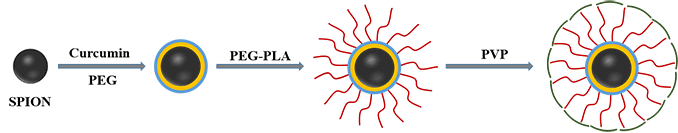
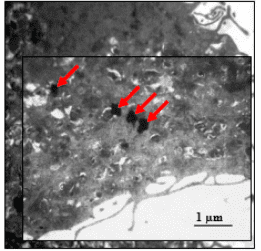
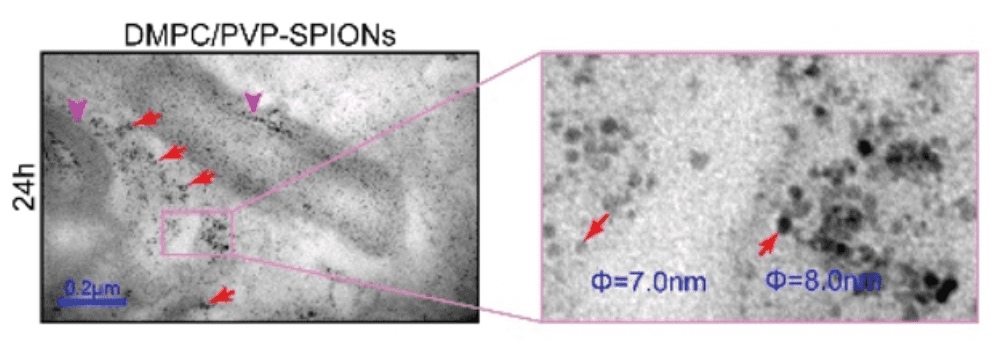
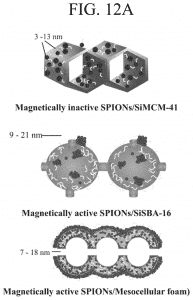
Nanoparticles, which have found applications in various biomedical fields such as drug delivery, biosensors, wound healing, etc., are now employable in a new field of research called “neuronanomedicine”, where nanomaterials are designed and engineered to interact with, and act as carriers of drugs to the biological systems in a molecular level. Due to the complex nature of the brain-blood barrier (BBB), better design and delivery of drugs to the brain to achieve maximum effectiveness is needed. To cross the BBB without loss or accumulation of blood, one method is to load the drug in nanoparticles (NPs) which can pass through the BBB. This combination of neurology and nanotechnology is a promising field for the treatment of central nervous system disorders, especially NDs (Neurological Disorders), because of the capability of nanoparticles to permeate through the BBB. Superparamagnetic iron oxide nanoparticles (SPIONs) seem to be promising target drug carriers due to their smaller size, which can easily pass through the BBB by encapsulation, external magnetic field, or local heating. However, stability, biocompatibility and cytotoxicity are their major challenges as drug carriers, which can be addressed by surface functionalization of SPIONs.
Introduction
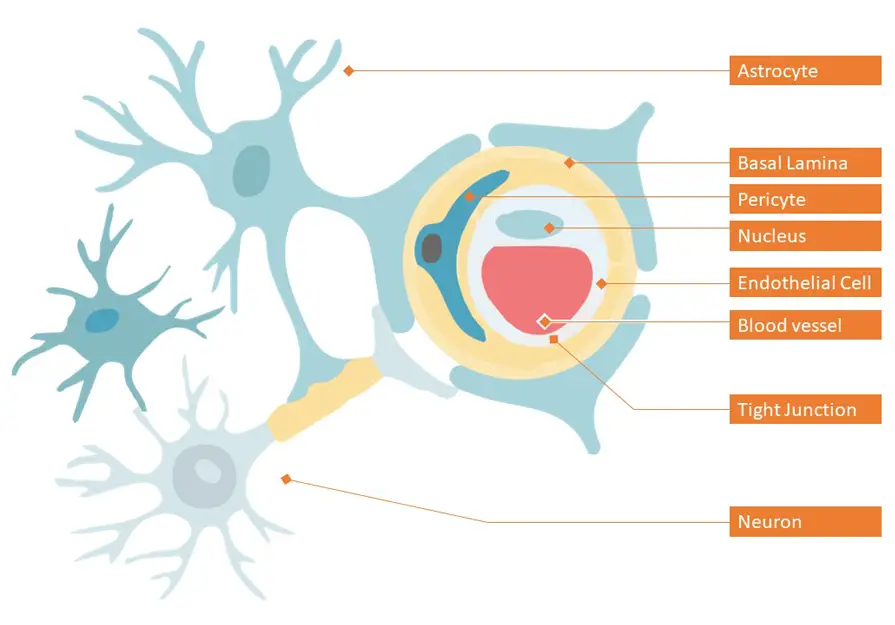
Dementia, a syndrome that impairs cognitive function, is ranked 7th among the leading causes of death worldwide. According to the latest report by World Health Organization (WHO) in 2020, dementia entered the top 10 in last 20 years, affecting millions of people worldwide.[1] This kind of neurological disease called neurodegenerative disease (ND) occurs when neurons in the brain or the peripheral nervous system slowly lose function and ultimately degenerate and decline.[2] Although medical science is well advanced today to tackle the diseases affecting the brain, the limitation for the treatment of neurological diseases is the difficulty in delivering drugs to the brain. Human brain is covered by Brain-blood barrier (BBB) which works effectively as a defense mechanism in preventing the majority of molecules, including drugs from entering into brain.[3] Figure 1 shows a representation of the BBB. The BBB protects the brain from toxic molecules and at the same time, maintains the brain homeostasis by regulating ion and nutrient transport.[4]