Introduction
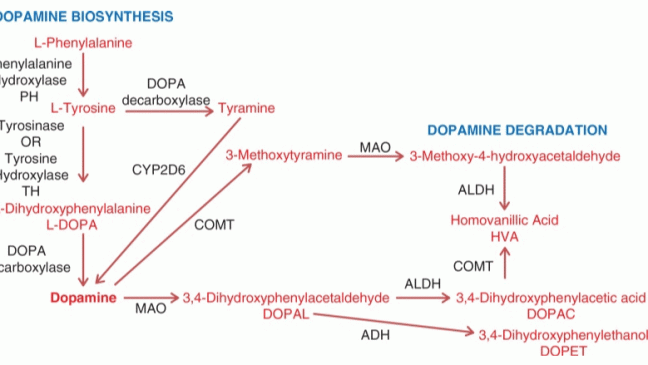
Parkinson’s disease (PD) is a neurodegenerative disorder that is characterized by the loss of dopaminergic neurons and accumulation of Lewy’s bodies leading to imbalance in the levels of dopamine. PD is the second frequent neuro disorder following Alzheimer’s disease. Symptoms of PD include tremors, bradykinesia, muscle stiffness, impaired posture and gait, loss of movement, changes in speech, changes in writing, thinking difficulties, constipation, depression, sleep problems, changes in blood pressure, smell dysfunction and pain and fatigue. The levels of norepinephrine at neuron ends, which control many automatic functions of the body are also affected, leading to non-movement features.[1] It is a slowly progressing neurodegenerative disorder with no exact cause identified, with symptoms similar to other neurodegenerative diseases such as multiple system atrophy, progressive supranuclear palsy, striatonigral degeneration. This makes it difficult to identify PD at an early stage.[2], [3]
Approximately 10 million people worldwide live with PD. The cost of PD is estimated to be close to $52 billion every year in the United States alone with nearly one million expected to be living with Parkinson’s disease by 2020. PD is generally diagnosed above the age of 60, with 1% of the population diagnosed during their 60s or later[4], [5].
The symptoms of PD are classified as motor and non-motor symptoms, with symptoms getting progressively worse as the disease develops.
- Motor Symptoms are the symptoms most strongly associated with the progression of PD. Motor symptoms include dyskinesia, dystonia, shuffling gait and micrographia.[6]
- Non-motor Symptoms include a loss in the sense of smell, sleep disorders and gastrointestinal issues such as constipation, all of which can occur years before the first stage of PD[7], [8].
Progression of PD
PD progresses in five stages, with non-motor and motor skills degenerating rapidly with the progression to the next stage.
Stage 1: Initially there are mild symptoms that do not affect normal life. Examples include tremors, changes in posture, and movement symptoms occurring on one side of the body.
Stage 2: Movement symptoms begin to progress to both sides of the body, with visible changes in gait and walking. Symptoms begin to affect daily routines, making them more difficult and time-consuming.
Stage 3: The movements become slower and there is more frequent loss in balance. While the patient is still able to live on his own, daily tasks such as eating become significantly impaired by PD.
Stage 4: Standing becomes difficult with patients requiring support of a walker. Symptoms are very severe, and patients cannot live alone.
Stage 5: This is the most advanced stage of PD, with little or no leg function. The patient experiences frequent hallucinations and delusions, and requires round-the-clock care[9].
Pathophysiology
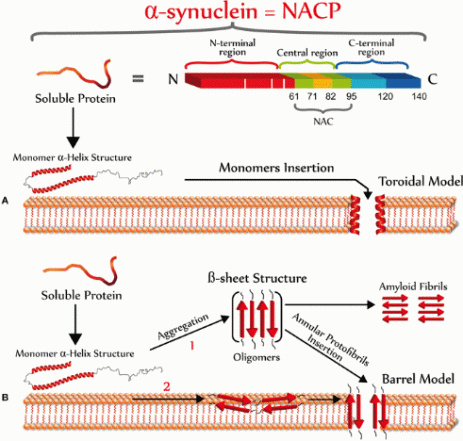
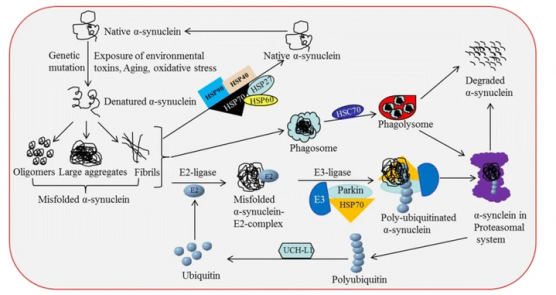
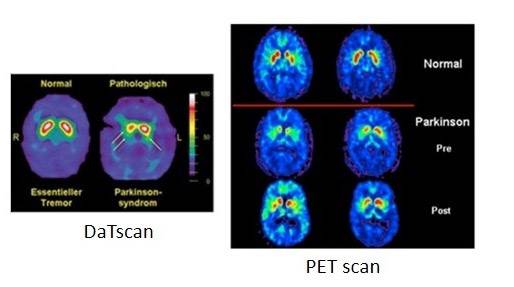
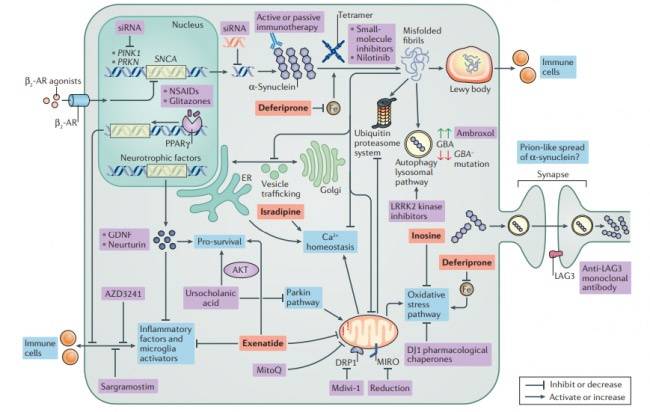
PD is characterized by a loss in dopaminergic neurons in the substantia nigra pars compacta, located in the midbrain. The pathological hallmark of PD is the aggregation of α-synuclein in the form of Lewy bodies and Lewy neurites, the underlying mechanism of which involves the following:
- Mitochondrial dysfunction
- Defective protein clearance mechanisms
- Neuroinflammation
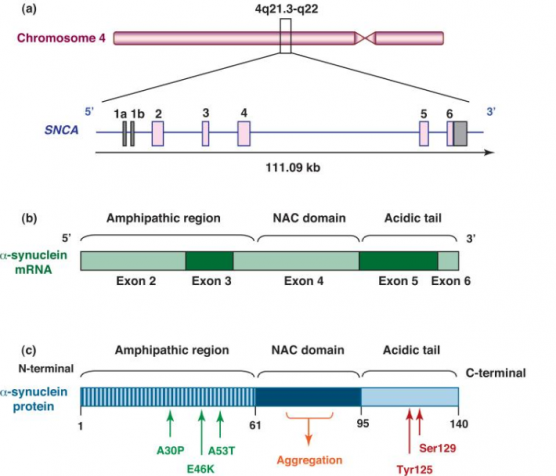
Structure of α-synuclein
α -synuclein is a presynaptic neuronal protein encoded by gene SNCA. The SNCA gene encodes a 140 amino acid protein which in aqueous solution is present as stable tetramers that resist aggregation. The protein does not have a defined tertiary structure. It is mostly unfolded, and is called as natively unfolded protein.
α -synuclein protein is composed of three distinct regions (Fig.1):
- An amino terminus with 1–60 amino acid residues, containing apolipoprotein lipid-binding motifs, predicted to form amphiphilic helices conferring the propensity to form α-helical structures on membrane binding.
- A central hydrophobic region with 61–95 amino acid residues, so-called NAC (non-Aβ component) which confers the β-sheet potential.
- A carboxyl terminus that is highly negatively charged, and is prone to be unstructured.