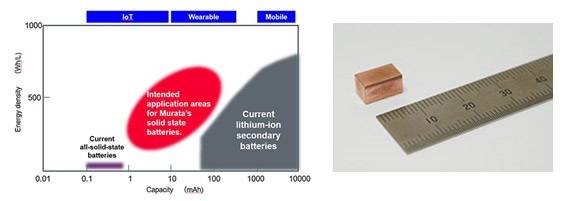
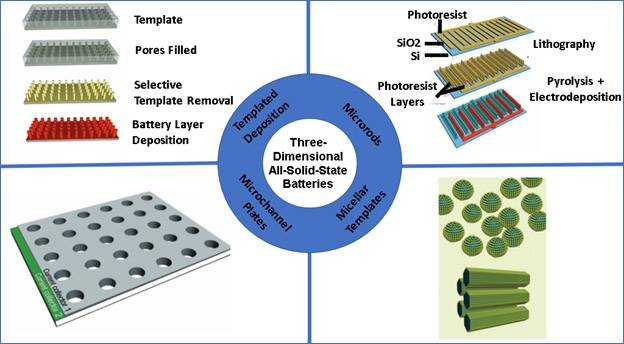
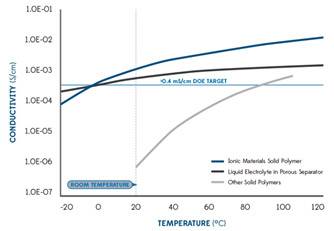
INTRODUCTION
Lithium-ion (Li-ion) batteries, containing liquid/gel separators, provide a lightweight energy-storage solution for most of today’s high-end battery-powered gadgets, ranging from smartphones to electric vehicles. Despite their prevalence, Li-ion batteries have disadvantages such as low power density, short lifespan and high resistivity at electrode interface, leading to capacity loss, electrolytic decomposition at high voltages that limit the use of high voltage cathode materials, formation of HF at thermal runaway, and risk of leakage, resulting in battery fires and explosions. Safety issues assumed great importance, following incidents such as an explosion in a Japan Airlines 787 Dreamliner’s cargo hold in 2013 and Samsung’s Galaxy Note 7 catching fire in 2016. The leakage of the liquid electrolyte, or short-circuit of the electrodes due to failure of the polymer gel separator was identified as the main cause for the explosion. Replacing the liquid/gel components of the conventional Li-ion batteries with solid electrolyte was considered as an approach to solving the problem.
Solid-state batteries use both solid electrodes and electrolytes. The emerging all-solid-state battery (ASSB) technology offers high performance and safety at low cost. ASSBs have low flammability, high energy density, high electrochemical stability and high energy density, as compared to conventional liquid/gel electrolyte batteries and find application in various industries such as energy storage, consumer electronics, industrial, aerospace and automotive.1 Currently, different types of solid-state electrolytes based on oxides, sulfides, phosphates, nitrile or polymers such as polyethers, polyesters, polysiloxane, polyurethane, etc., exist. 2,3,4
Though promising, ASSBs still face challenges with respect to solid interface stability, large scale production, sustainable manufacturing, recyclability, and so on. Although great potential exists, there is still plenty of efficiency to be gained by improving the chemistry of lithium-ion batteries. 5,6
RECENT DEVELOPMENTS
Researchers at the Samsung Advanced Institute of Technology (SAIT) and the Samsung R&D Institute Japan (SRJ) proposed a silver-carbon (Ag-C) composite layer as the anode to replace lithium metal anodes that are generally used in ASSBs. The 5µm ultrathin Ag-C nanocomposite layer incorporated within a prototype pouch cell enabled a larger battery capacity, a longer lifespan and enhanced safety. Such a single charge of the prototype pouch cell may enable an electric vehicle (EV) to travel up to 800 km (500 miles), while offering a lifecycle of more than 1,000 charges. The Ag-C nanocomposite layer also helped in the reduction of anode thickness while boosting energy density up to 900 Wh/L. 7