Introduction
Cancer cells have characteristics of genetic variabilities and accumulate somatic mutations rapidly. The genome sequencing of cancer cells has identified heterogeneity and tens to thousands of somatic mutations that vary among individual patients. Nonsynonymous somatic mutations may alter the amino acid coding sequences and create abnormal proteins that become highly expressed and promote cell proliferation. Tumor-specific antigens (TSAs), also known as neoantigens, are created by the process of genomic codon interchanges, editing, antigen processing and presentation. Neoantigens can be presented by the major histocompatibility complex (MHC, also known as human leukocyte antigen (HLA) in humans) on the cell surface and recognized by the T lymphocytes. They are tumor-specific and not expressed by normal cells, which makes them ideal therapeutic targets. Targeting neoantigens has the potential to maximize therapeutic specificity, while minimizing the risk of autoimmunity.
Evolving Immunotherapies for Cancer Treatments
In recent years, immunotherapies have advanced a new era of cancer treatment. In 2011, FDA first approved an immune checkpoint inhibitor (ICI), ipilimumab, a monoclonal antibody targeting CTLA-4, which extended the overall survival rate of patients with metastatic melanoma. Developed as the next generation of immunotherapy, it uses personal and precision vaccines as well as T cell therapies to direct T cells directly toward a patient’s tumors. In 2018, Dr. James P. Allison was awarded the Nobel Prize in Medicine for his work on cancer therapy by inhibition of negative immune regulation. There are other (or newer) ICIs (such as anti-PD1 and anti-PD-L1 antibodies), considered to be effective therapies in subsets of patients with a variety of tumor types such as metastatic melanoma, non-small cell lung cancer (NSCLC), prostate cancer, renal cell carcinoma, and so on. However, the use of ICI carries a risk to develop immune-related adverse events, which occur via activation of the patient’s immune system, leading to serious and even fatal reactions. Additional efforts are needed to improve the response rates and tumor antigen specificity of ICI, and address the incidence of immune-related adverse events. The first chimeric antigen receptor- (CAR-) T cell immunotherapy, anti-CD19 CAR-T for B cell lymphoma, was approved by the USFDA in August 2017. Subsequently, there has been an increasing number of clinical trials using CAR-T therapy to treat cancers. CAR-T cells target the tumor-associated antigens (TAAs), such as CD19 in B cell malignancies and ERBB2 in breast cancers, which are also expressed in normal cells. While CAR-T therapies have shown significant promise in acute lymphoid leukaemia, treating solid cancers with CAR-T cells remains a challenge due to the lack of suitable tumor-associated antigens and low overall objective response rates.
Neoantigens have been considered an important therapeutic approach to cancer treatment. Neoantigens could be represented by HLA and recognized by T lymphocytes of the immune system. The presence of such cancer neoantigen recognizing T cells has been associated with effective antitumor immunity in humans. Neoantigen-specific T cells are crucial to clinical responses. The isolated T cell clones or T cell receptor (TCR-) engineered T lymphocytes establish the epitope patterns of neoantigens recognized by T cells. There are increasing neoantigen-based cancer vaccines designed to target the unique immunogenic mutations in each patient’s tumor. Recent success of personalized cancer vaccines can be attributed to the RNA mutanome vaccine and peptide-based vaccine induced by poly-specific therapeutic immunity. Neoantigen cancer vaccines are capable of eliciting strong T cell responses to neoepitopes in patients with melanoma. Neoantigens arise from non-synonymous mutations and other genetic alterations in cells. Neoantigens are mutated peptides present as HLA on the cell surface, and theoretically more attractive therapeutic targets because they are different from the others and seen as non-self by the immune system. Normal cells do not express Neoantigens and therefore, neoantigen-specific immune reactions are not subjected to central and peripheral tolerance. Several neoantigens have been identified for different types of cancers, including melanoma, lung cancer, hepatoma, and renal cancers. Neoantigen vaccines and immune checkpoint inhibition act as complementary treatments that might often be used together, especially for patients with large tumors or metastatic disease, and can lead to an increased tumor-specific immune response.
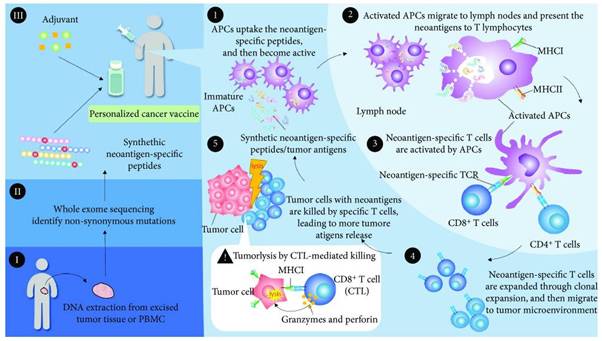
Mechanism of action in Neoantigen-based Cancer Vaccines
In order to generate personalized cancer vaccine, somatic mutations within cancer cells can be identified using whole exome sequencing. Based on the mutations identified, a personalized cancer vaccine can be designed to target the specific epitopes of mutated neoantigens, and may consist of synthetic peptides or genes encoding the shared tumor antigens, accompanied by the presence of adjuvants such as poly-ICLC, GM-CSF and BCG.
The neoantigens from the cancer vaccine or dead cancer cells are captured by antigen present in cells (APCs). Next, the activated APCs migrate to the lymph nodes and the MHC molecules present the neoantigens to T lymphocytes. The specific TCR recognizes the neoantigens, resulting in the priming and activation of T cell immunity. Neoantigen-specific T cells expand, traffic and infiltrate the tumor microenvironment. The expanded T cells specifically bind to the neoantigens of cancer cells via the interaction of the TCR/neoantigen/MHC complex. The CD4+ T cells augment the immune response against cancers, and CD8+ cytotoxic T lymphocytes (CTL) directly kill the cancer cells through the degranulation of granzyme, granulysin or perforin. The lysed tumor cells release more neoantigens, which produce the adaptive immune memory response and lead to the expansion of molecularly heterogeneous T cells against cancers.