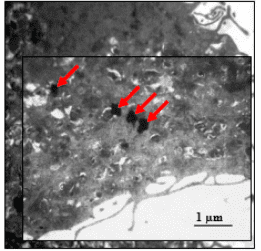
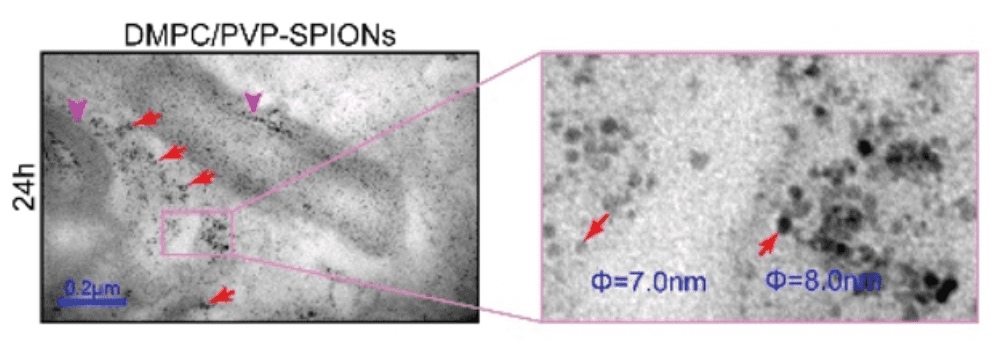
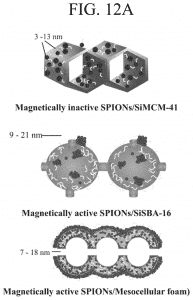
Introduction
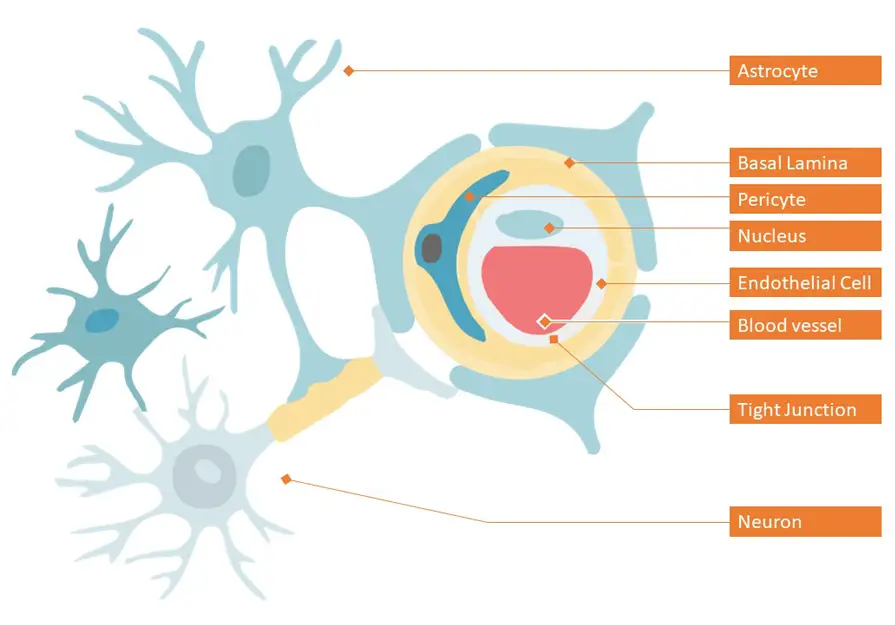
Dementia, a syndrome that impairs cognitive function, is ranked 7th among the leading causes of death worldwide. According to the latest report by World Health Organization (WHO) in 2020, dementia entered the top 10 in last 20 years, affecting millions of people worldwide.[1] This kind of neurological disease called neurodegenerative disease (ND) occurs when neurons in the brain or the peripheral nervous system slowly lose function and ultimately degenerate and decline.[2] Although medical science is well advanced today to tackle the diseases affecting the brain, the limitation for the treatment of neurological diseases is the difficulty in delivering drugs to the brain. Human brain is covered by Brain-blood barrier (BBB) which works effectively as a defense mechanism in preventing the majority of molecules, including drugs from entering into brain.[3] Figure 1 shows a representation of the BBB. The BBB protects the brain from toxic molecules and at the same time, maintains the brain homeostasis by regulating ion and nutrient transport.[4]